All (or almost) you need to know about coal
In the realm of energy, few resources have ignited as much controversy as coal. For centuries, this black, carbon-rich fossil fuel has played a vital role in powering human progress, fuelling industrial revolutions, and shaping the modern world. Yet, with the ceaseless march towards a greener future, coal finds itself at the centre of a heated debate, caught between its historic significance and its dramatic environmental implications.
In the context of Energy Observer’s stopover in South Africa where 85% of the electricity is produced out of coal, our onboard scientist and energy expert Beatrice Cordiano will try to answer the following: Where does this fossil energy comes from? Could a clean coal production exist? Let's figure it out!
The coal family: tracing back millions of years
Even if early mines were documented in ancient China and the Roman Empire, the modern history of coal begun 250 years ago, at the beginning of the Industrial Revolution, when some British inventors discovered it could be used to heat water, make steam and spin turbines to power machines. It was a cost-effective substitute for wood, delivering more energy when burned. Since then, coal has become an indispensable resource, widely used for power generation, residential heating and to produce the concrete and steel our lives are built around. But coal is way more ancient than that. Its origins date from millions of years ago, back when carbon-rich plants died in swamps and over time the combined action of pressure and heat turned them into black and shiny rocks packed with energy.
Coal mining in the British Industrial Revolution
These altered remains of prehistoric vegetation come in various forms, each with its own unique traits, composition and energy density. From peat and lignite to sub-bituminous, bituminous and anthracite: coal is ranked according to its evolution over time and, therefore, its carbon content. The deeper the coal seam, the longer it took it to form, the higher its purity and rank. This is due because at deeper depths the material encounters greater temperatures and pressures, and more plant debris is transformed into carbonaceous structures. Therefore, the older the coal, the higher its quality.

Komati Power Station in South Africa
Peat is coal in its youthful stage of formation, it is the precursor of coal, an accumulation of partly decayed vegetation that has gone through a small amount of carbonization - a process that takes place under incredible heat and pressure - but can eventually transform into coal under the right circumstances. Next in line, at the lowest rank of the coal’s family, there is lignite, a crumbly brown rock presenting low amounts of carbon (50-60 %) and therefore little energy. Sub-bituminous follows, which is coal in its intermediary state, showing a balance between energy and purity (60-70%).

Kusile in Joburg
Bituminous comes after, the backbone of the coal family and, formed under more heat and pressure, it contains 70 to 90% carbon, which makes it a popular choice for industrial use. Anthracite, the premium coal, boasts the highest carbon content (up to 97%) and energy density and burns cleanly. In fact, the higher is the quality the cleaner the combustion, the fewer the emissions. And eventually graphite, the result of the final stage of carbonization and an allotrope of carbon, namely a substance consisting solely of carbon atoms, that can be found in pencils and is commonly used in lithium-ion batteries thanks to its great conductivity properties.
Coal and oil, the dream team
Today, coal is both the second most important source of primary energy and the largest source for electricity generation - accounting for 27% and 36% of the global mix respectively - and the most polluting fossil fuel. But there is one source which is used almost as much as coal: oil. Coal and oil are like two sides of the same coin, each possessing unique strengths that complement one another. Coal is cheap, is abundant, oil is more versatile, but harder to find. Coal provides stability and reliability powering the grids, it can be used as a raw material in the production of steel, iron and chemical products and, in countries with little access to clean fuels for cooking, it is often used in the kitchens. Oil, on the other hand, is used to make plastics, pharmaceuticals, synthetic fabrics and, more importantly, it has been the ideal fuel for transportation so far thanks to its liquid form and energy density, averaging twice the one of coal by weight.

Energy density and CO2 emissions from fuel combustion
The dirtiest source of energy
All living organisms consist primarily of carbon-based molecules. Thus, fossil fuels, deriving from once-living matter, also contain carbon. Regardless of the fuel, they must be burned to generate energy and the heat content of a certain fuel, or in other words, the amount of energy released during combustion, is mainly determined by their carbon and hydrogen content. Here’s where the carbon and hydrogen which are stored inside are released as carbon dioxide and water vapor. Also, the more carbon is chemically found inside the fuel, the higher the carbon emissions.
Burning coal produces about 15 billion tonnes of CO2 each year. But that’s not all: CO2 is not the only by-product of its combustion and that is the reason why coal is the dirtiest source of energy. Sulphur dioxide (SO2) and nitrogen oxides (NOx) are released contributing to air pollution, respiratory illnesses, and acid rains. On top of that, there are mercury - a toxic metal which, when dissolved into water, accumulates in ecosystems posing a threat to aquatic life and human health - and particulate matter that, depending on the coal composition, can contain toxic and irritant elements such as cadmium, silicon dioxide, arsenic and calcium oxide. If you ever wondered what coal combustion brings along, here it is.
Would a « clean coal » be possible?
As countries commit to move away from this source of energy, its use continues to prevail in some emergent countries like China, India, Indonesia and South Africa as a major component of the energy mix. Of the 30 most coal-reliant countries only about half have committed to phase-out coal and six countries have neither set targets to abandon this source nor to reach net zero.
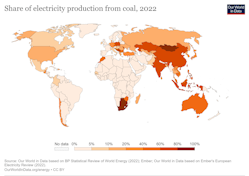
Our World in Data based on BP Statistical Review of World Energy (2022)
It has been estimated that to meet climate goals, we would need 117 GW of generation from coal to be retired every year, yet in 2022 only 26 GW were retired and to add a little complexity, as natural gas prices surged due to the Ukraine war, many European countries lifted caps on coal generation. We are clearly not on track to reach the Paris agreement’s targets and the reason is that coal, despite its poor environmental performances, is abundant, efficient and less expensive than most other energy options.
Attempting to use this energy source without adding to atmospheric emission levels is a major challenge and efforts are underway to reduce the impact of coal-fired power plants and to develop “clean coal” technologies. Acting upstream or downstream, the solutions are diverse. It is possible to enhance the efficiency of coal-fired power plants through supercritical and ultra-supercritical combustion, which require less coal per megawatt-hour, maximizing the energy output while minimizing emissions. Another possibility is to add electrostatic precipitators or scrubber systems to trap suspended particles and significantly reduce SOx, NOx and particulate emissions and use abatement technologies like carbon capture units to permanently store the CO2.
Many solutions are available, all of them are possible and can help reduce the emissions from coal but, let’s not forget, none of them can be better than reducing coal dependence and diversifying the energy mix by integrating renewable energies.