Water, the molecule of life
For this International Water Day, our scientist, Dr. Katia Nicolet, has geeked out on water, this wonderful, magical molecule that is so central to our lives that we take it for granted. But we most definitely should not. Learn more about water today and never see a tall glass of water the same way ever again.

Sargassum in the Atlantic Ocean
The molecule of life
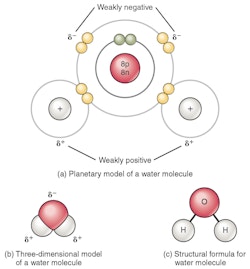
The water molecule has some unique properties that allow for life on earth - or rather, life evolved based on these special qualities of water. Either way, water is synonymous with life on our Blue Planet. Water is comprised of one atom of oxygen (O) and two atoms of hydrogen (H), together they form an overall stable and electrically neutral molecule (it’s not charged). However, and that’s where things get interesting, because electrons prefer to be closer to the oxygen atom than the hydrogen atoms, the molecule ends up having a Y configuration, with the two hydrogen atoms being closer together on one side.
As a result, one side of the molecule is slightly positively charged, and the other slightly negatively charged. This polarity (slight positive/negative charge) allows the molecule to behave like a magnet being “attracted” to other water molecules or other polarised molecules. The weak bonds between the hydrogen of one water molecule and the oxygen of another water molecule is called a Hydrogen bond, and it makes water almost magical.
Due to its polarity, water is a powerful solvent! For example, the molecule of salt (NaCl) gets pulled apart by water because the Na+ gets attracted to the negative side while CL- is attracted by the positive side. Salt is thus dissolved in water, allowing for the salinity of our ocean. This is also how water can dissolve minerals in rocks and sediments and become an important source of minerals for humans, animals and plants. 20% of our calcium and magnesium daily intake, for example, comes from drinking water (Olivares M. & Uauy R., 2005, Essential nutrients in drinking water, World Health Organization).
Ice floats, no big deal, right?
The hydrogen bonds between water molecules result in another property called cohesion. Cohesion means water molecules stick together which, in turns, allow for capillarity. And capillarity is how water will happily flow through plant tissues and blood vessels even against the force of gravity. Magical. Or physics. You decide. Cohesion is also responsible for surface tension, the reason, for example, that water forms a dome at the surface of a slightly over-filled glass or that water striders (insect) can walk on water without breaking its surface.
But the craziest thing of all: that extra stickiness means that water is liquid, rather than a gas, at room temperature because of the hydrogen bonds! Although the hydrogen bonds are continually forming and breaking as the water molecules move, there is enough stickiness to keep water molecules close together and prevent it from being a gas at 20˚C. Furthermore, the bonds in liquid water keep the molecules very close together, but when water freezes, the molecules arrange themselves into a crystal, creating more spaces between the molecules. This is why liquid water is denser than solid water.

Glacier in Spitsbergen
And this is immensely important. We’ve always seen ice float on water, so we’ve never really questioned it, but can you think of any other element that is denser as a liquid rather than a solid? Does solid lead float on liquid lead? Does solid oil (fat) flat on liquid oil? No!, because the liquid state usually means the molecules are more excited, that they move around more and that, thus, there is more space between them. But not water. The densest water gets is actually at a temperature of 4˚C, and the least dense -you guessed it- at 0˚C. Magical, again.
Life and death due to ice
So, ice takes more space than water. You’ve probably experienced it when the water bottle you left in the freezer exploded, or when a water pipe burst over winter. This property of ice has important consequences for the weathering of matter and the creation of sediments through erosion. It is also why most organisms die when they freeze, because the ice formation within the cells pierce the cell’s membrane. Only a few animals and plants with antifreeze proteins can survive their body temperature dropping under 0˚C. And it’s also the reason why, if you were cryogenized, you’d turn into a puddle of blood and tissues once you thawed. Unless you’re a cool Antarctic fish.
Less dense ice also means life, however. If solid water was denser than liquid water, lakes, rivers and oceans would freeze from the bottom up. As soon as ice crystals would form, they would sink and accumulate at the bottom, until everything froze over. All the diversity of bacteria, plants, invertebrates, fish and amphibians that live in lakes and in the polar oceans would die off every year, greatly impacting the aquatic biodiversity. Instead, the ice forms at the surface, adding a moderately cold layer (0˚C) between the water and the often colder conditions of the environment. The aquatic organisms are thus sheltered from freezing temperatures and can survive until spring.
The Blue Planet

Diving under sargassum
It is probably clear to you now that water is a very unique molecule and that life on earth depends on it. Actually, 70% of the planet is covered by oceans! But for us, non-marine organisms, salt water is not drinkable. If we were to drink salt water, the water in our cells would try and dissolve the salt water by a process called osmosis, and we would end up gravely dehydrating ourselves. Same thing for non-marine plants and animals. As a result, terrestrial and freshwater organisms depend on, well, freshwater. And there is surprisingly little freshwater on earth!
Of all the water present on earth, 97.5% is saline water! That leaves a small 2.5% percent of freshwater on this planet (approximately 34 650 000 km3). But wait! Of all the freshwater, about 69% is stored away in glaciers, ice caps and frozen grounds (permafrost; numbers are rounded). So only 31% of the freshwater on earth is actually available for life, the large majority of which (30%) is groundwater, and only 1% of all the freshwater in the world is surface water (435 100 km3).

Importance of groundwater
Groundwater is all the water that infiltrates and percolates through the ground to reach underground aquifers. These aquifers consist of different materials (sand, gravels, permeable sedimentary rocks, volcanic and crystalline rocks) that trap the water from surface precipitation and snowmelt. Even if surface water is what we can see and experience every day, groundwater actually provides most of the water the global population uses (agriculture, farming, industry) and drinks. In some arid regions, it is the only available source of freshwater, resulting in 2.5 billion people depending solely on groundwater as a source of daily water.
This storage of underground water is also immensely important for the habitats and vegetation living at the surface. It plays a key role in keeping the water level and flow into rivers, lakes and wetlands, especially during drier months. This water flow through the ground is essential for the wildlife and vegetation, with only desiccation resistant plants being able to live on areas removed from groundwater (some elevated regions).
Consumption and pollution of groundwater
Our water needs are difficult to grasp, but to support the diet of an average American, by growing crops and raising animals, 5 000 litres of water are needed per day! Globally, irrigation accounts for more than 70% of total water withdrawal, both surface and groundwater. And about 45% of the total irrigation water use comes from groundwater. And this is only for the human population needs, animals and ecosystems require extensive amounts of freshwater to sustain biodiversity and life. Freshwater ecosystems are home to a variety of plants and animals, and these ecosystems are currently experiencing an extinction rate up to 15 times greater than marine ecosystems! For all these reasons, freshwater is immensely important.

Spitsbergen
Unfortunately, freshwater systems, and groundwater in particular, are threatened by over-consumption, degradation and pollution. Wells have emptied entire aquifers where the water needs of the human population exceeded the input of water from precipitation. Reducing the level of some aquifers close to the ocean sometimes results in salt water infiltrations within the porous ground, rendering the groundwater undrinkable and unusable for crops. Lastly, all sorts of man-made pollutants can infiltrate the ground along with precipitation and contaminate groundwater reservoirs. A non-exhaustive list includes pesticides and fertilizers from fields, salts from roads, chemicals from landfills, organic pollution from leaky septic and sewage systems etc.
Better management
Freshwater is a finite resource on our planet and we, and hundreds of thousands of species, rely on it for survival. As climate change worsens, groundwater will become even more critical. We need a more sustainable management of groundwater systems worldwide. On this International Water Day, we need to “make the invisible visible” and ensure that groundwater, although out of sight, does not remain out of mind.